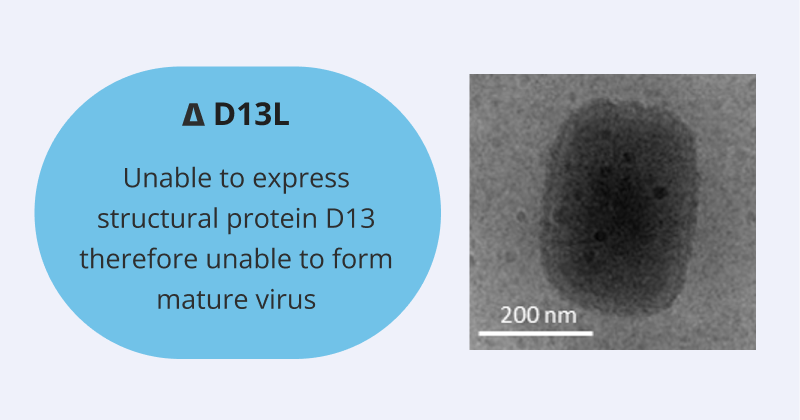
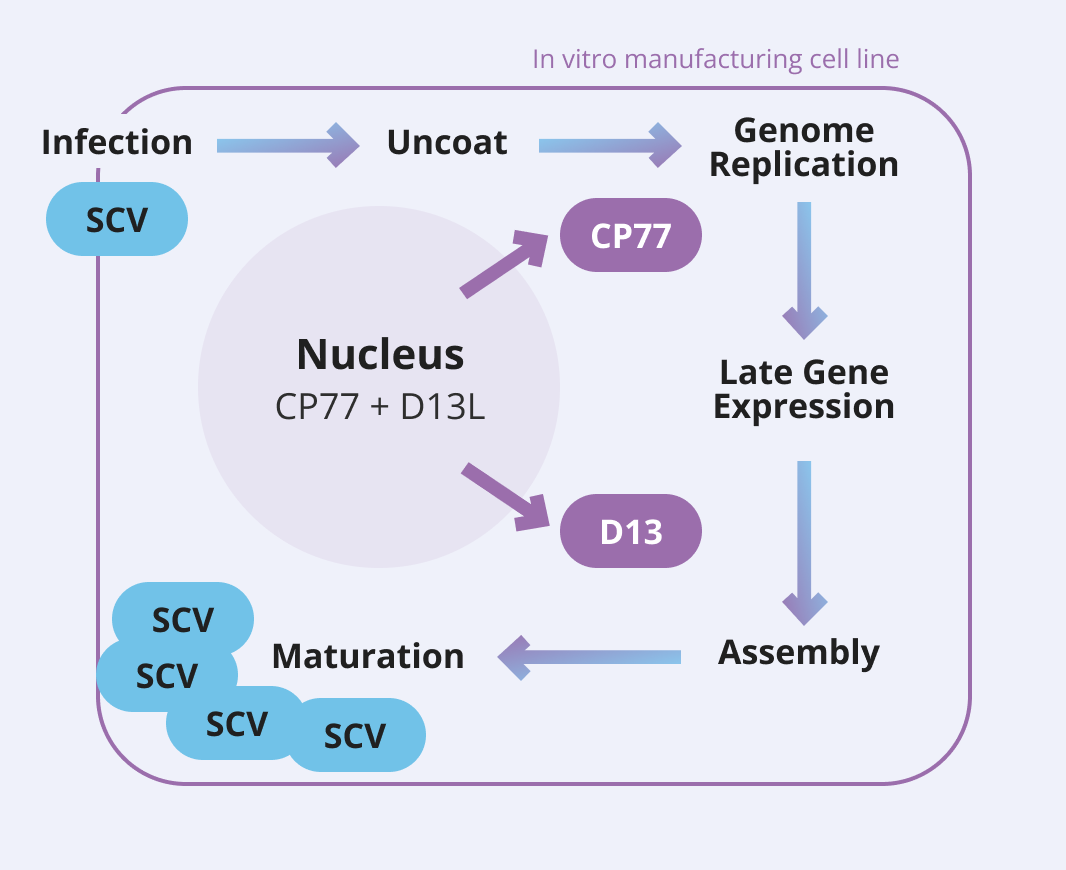
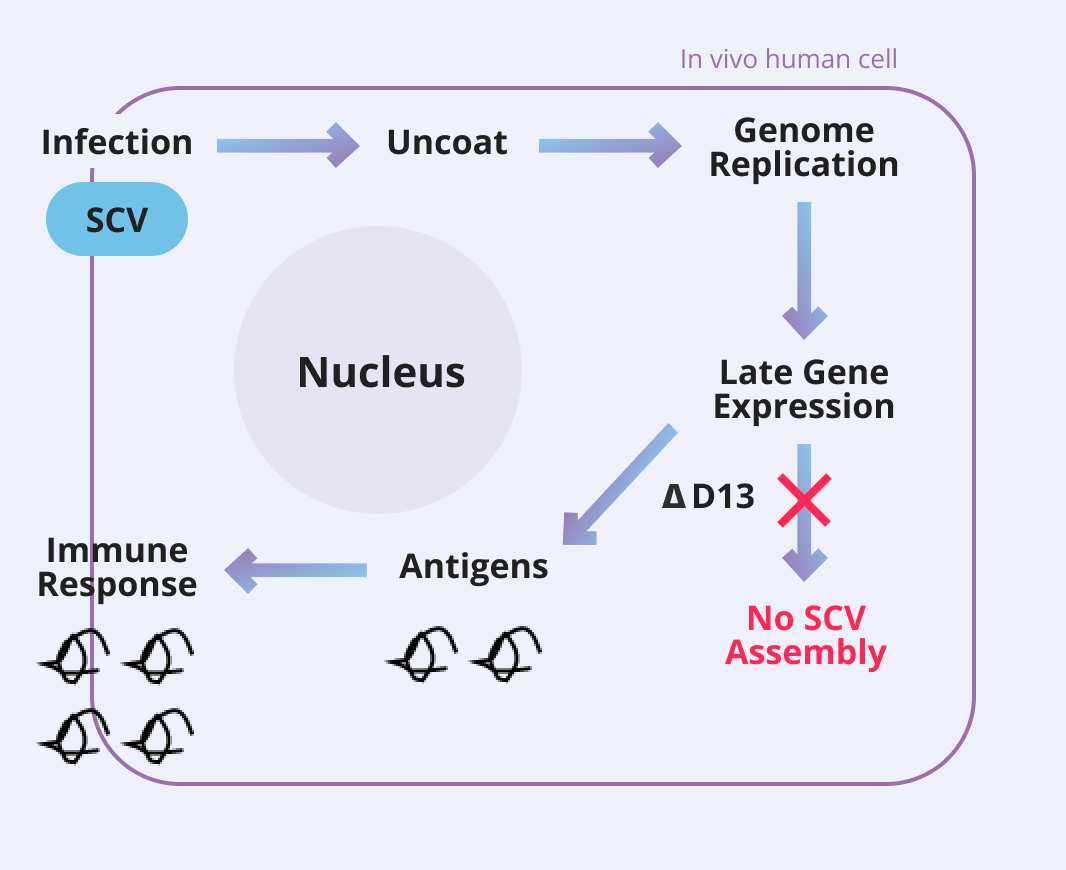
The SCV Vaccine Platform
Sementis was founded in 2009 with the aim to improve on the MVA-BN viral vector and the overall success of vaccinia vectors by overcoming:
- Manufacturability (complexity, scale, and lead times)
- Perceived immunogenicity limitations
The core technology is protected by a comprehensive patent portfolio and consists of two key elements.
- A fourth-generation vaccinia vector
“Sementis Copenhagen Vector (SCV)” - An advanced manufacturing platform
The SCV Vaccine Platform is built on more than 10-years of research to establish the platform. An extensive preclinical data package supports its broad utility including demonstration of the ability to generate strong, broad, and long lasting protective immune responses in animal models, and the ability to protect against disease in higher order animal challenge models.
The SCV Vaccine Platform is ready to enter Phase I clinical trials.